Regenerative medicine・Cell theraphy
The market of the regenerative medicine industry continues to grow worldwide.
Pharmaceutical pricing of approved cell therapy products are very high, and there are many
issues for generalization of regenerative medicine. Especially, there are cases that culture
medium occupai half costs of drug prices of cell therapy products. Additionally, conventional
culture medium contain animal-derived materials such as serum, which have safety issues.
Myoridge supports to solve regenerative medicine issues utilizing our technologies, media
component libraries and media optimization.
Examples of achievements
-

Regenerative medicine start up company
Optimization of culture medium composition for regenerative medicine applications of mesenchymal stem cells developed by user company.
-

Chemical manufacturer
Activity evaluation of non-animal-derived cytokines using various cytokine-dependent cell lines.
-
Machine manufacturer
Support for the development of mass culture equipment by examining iPS cell culture protocols and differentiation induction protocols.
Do you have any of these concerns about the culture medium?
-
Difficulty in reproducibility
Lot-to-lot differences in results due to serum components. Lot-to-lot effects of growth factors, cytokines, etc.

-
High Cost
Expensive sera and cytokines put pressure on research costs.

-
Selection of culture medium and serum
When culturing multiple cell cultures, it is difficult to select the best conditions for individual cells.

Medium optimization is your solution!!
Medium development contract service
We ask your issues of culture medium, and we will perform culture medium optimization that consists of proposal of culture medium, screening of culture medium, and consideration results of experiments. In the result, the optimized medium will be decided. Contract of medium development is performed by setting milestone by steps.
Milestone image
-
Step
1Making cell proliferation and cell surface markers as evaluation indicators.Example) To be same or more than conventional medium for level of cell growth and expression of surface makers. -
Step
2Making cell function and cell morphology as evaluation indicators.Example) Gene expression level that you specify fulfill your standard. -
Step
3Supply of optimized medium and medium composition disclosure.Example) We disclose optimized medium composition, we produce and supply according to the order.
List of medium development achievements
| Cells | results |
|---|---|
| iPSC derived cardiomyocytes | We achieved medium development of completely protein free by small molecules, improving of purity of cardiomyocytes. |
| iPS cells | We developed low cost medium improved cell proliferation and adhesion. |
| MSC | Compared to conventional medium, we achieved low cost, improved cell proliferation and serum-free |
Development period
Our medium optimization service can significantly shorten development period.

To cell product CDMOs(Contract Development and Manufacturing Organizations)
Culture medium that accounts for a lot of cost influences cell function.
There are cases that need to improve medium composition because of animal derived materials.
If you use our medium optimization, you can shorten development period
and increase success probability development of users that you support.

-
Evaluation or new components of culture medium, and development consulting

For new culture medium components developed by your company (peptides, non-animal-derived proteins, low-molecular compounds, etc.), we will select cell types suitable for evaluation and conduct everything from constructing an evaluation system to screening medium components.
ExampleTest system Select component-dependent cell types Evaluation substance Developed product, commercially available control product Evaluation item Cell growth, Surface maker, Activity of function -
Evaluation of Culture substrate and development consulting

We will ask you about the characteristics of the new material your company is developing, and advise you on the direction of development based on the latest information and our own knowledge in the field of regenerative medicine and cell therapy. In addition, we will evaluate the performance of the culture substrate developed by your company as a culture substrate using various types of cells.
ExampleTest system iPSC, MSC etc. Evaluation substance Developed product, commercially available control product Evaluation item Cell growth, Surface maker, Activity of function Protocol Cells are seeded onto various culture plates, and 72 or 96 hours later, cell counts and immunostaining are performed to evaluate surface marker expression levels and differentiation potential.
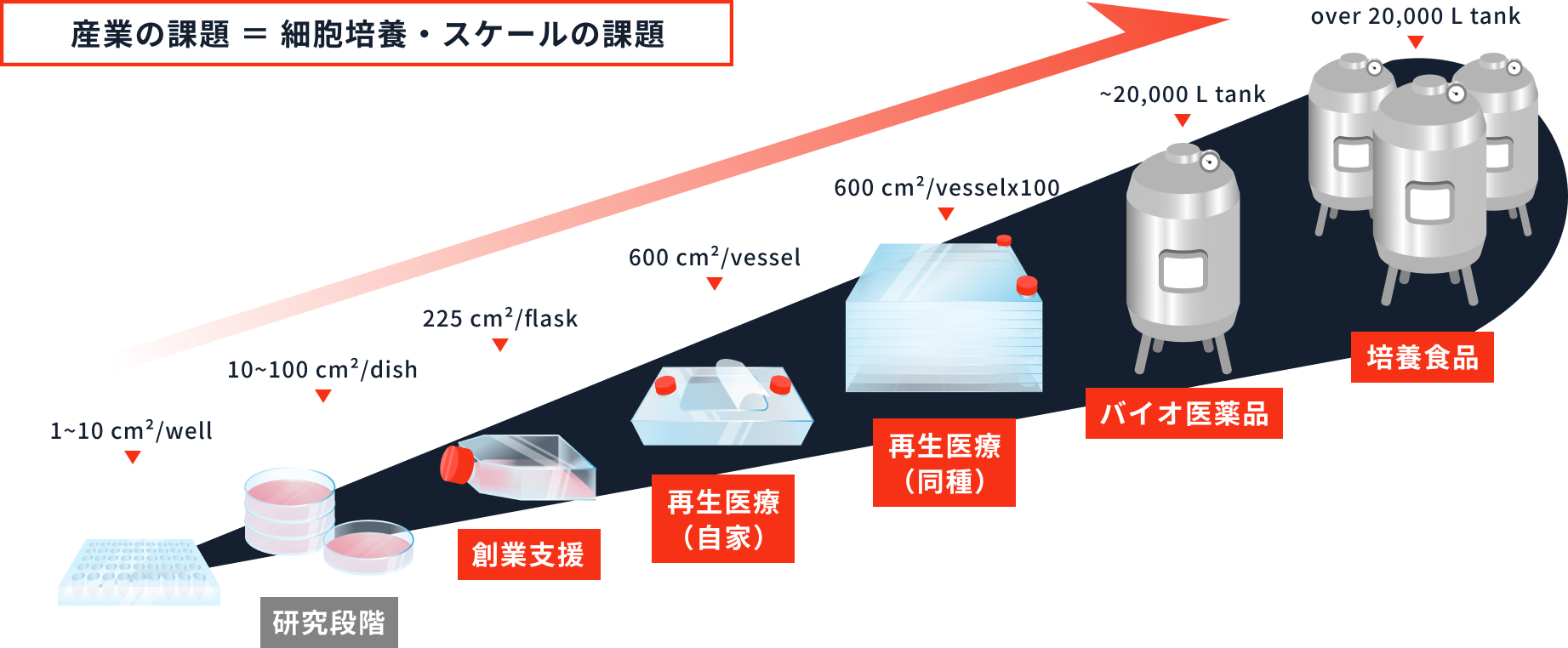
Development and introduction support for automatic culture and mass culture equipment
When scaling up from laboratory-scale culture to manufacturing scale, the environment for cells changes significantly, so it is not easy to reproduce the cell proliferation and functions observed at laboratory scale during scale-up. In addition to developing culture medium to accommodate changes in the culture environment, we also support the development of protocols for transitioning to mass culture or automatic culture. We collaborate with companies that develop automatic culture and mass culture equipment to develop culture media and culture protocols to meet the user's cell culture requirements.