List of Examples of achievements
Optimization of culture medium at the regeneraive medicine
-
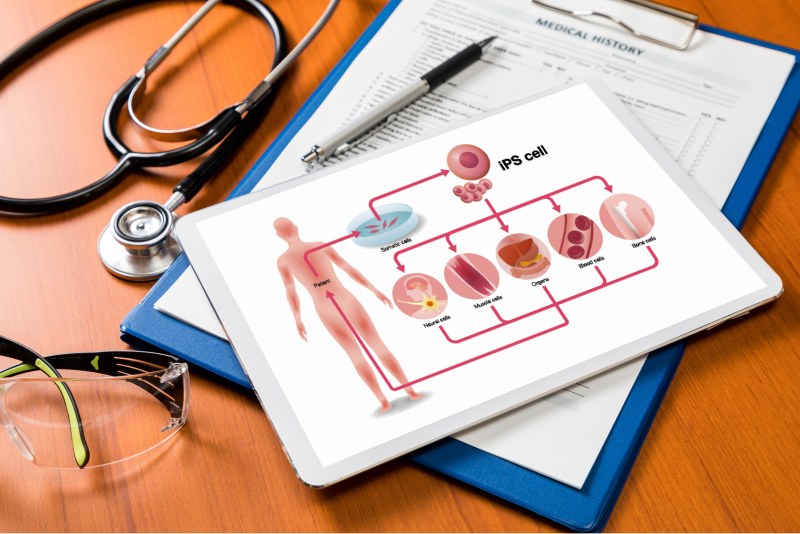
Regenerative medicine start up company
Optimization of culture medium composition for regenerative medicine applications of mesenchymal stem cells developed by user company.
We received user's cells. We produced test medium, and performed first screening using proliferation and surface marker expression as evaluation indicators. At the second screening we evaluated cell function, and decided candidate medium. User performed cell culture using candidate medium. After confirming reproducibility, we disclosed composition of successful medium for user.
-
Chemical manufacturer
Activity evaluation of non-animal-derived cytokines using various cytokine-dependent cell lines.
We evaluated cell proliferation independent of medium compounds that user developed at the first screening. If result of the first screening indicated high activity compare with other company's products, we evaluated some function of cells at the second screening.
-

Machine manufacturer
Support for the development of mass culture equipment by examining iPS cell culture protocols and differentiation induction protocols.
We investigated the culture protocol of iPS cells using mass culture equipment that user developed. While of monitoring each parameter in the culture medium, we supported development of equipment and making culture protocol about timing of changing medium and way of passages.
Other case studies
-
Regenerative medicine start up company
Optimization of culture medium composition for regenerative medicine applications developed by user company.
-
Overseas regenerative medicine start up company
Licensing and technology transfer for completely protein-free differentiation induction method from iPS cells to cardiomyocytes and clinical development.
-
University
Supply of custom Midium for smooth muscle cell culture.
-
Chemical manufacturer
Comparative study of cell culture materials made from new materials with commercially available materials using mesenchymal stem cells and iPS cells.
-
Chemical manufacturer
Performance evaluation of media introduced by user company. (Cell proliferation, Differentiation ability, stc.)
-
Machine manufacturer
Examination of new culture processes in Automatic cell culture system manufactured and sold by user company.
-
Food manufacturer
Examining of application to cultured meat culture medium using by-products of food manufacturing.
in vitroOptimization of culture medium at the development of test model
-

Pharmaceutical company
Development of heart failure model using iPS cell-derived cardiomyocytes.
We performed reproduction experiment of the paper using iPS cells derived cardiomyocytes, and we evaluated activity of positive control drug. After reproduction experiment, we considered disease model construction of gene edited iPS cells derived cardiomyocytes.
-

Pharmaceutical company
Differentiation Induction of cardiomyocytes from genome-edited iPS cells.
We received iPS cells owned by the user, we performed differentiation induction to cardiomyocytes follow the steps below. ① Confirmation of differentiation possibility, ② Confirmation of yield and drug responsiveness of differentiation induced cells, ③ Production of differentiation induced cardiomyocytes
-
Drug discovery start up company
Development of mitochondrial ATP sensor-introduced cells and drug screening method targeting mitochondria.
We received iPS cells owned by the user, we introduced mitochondrial ATP sensor into iPS cells. After selection of stable expression lines, we performed differentiation induction to cardiomyocytes, and made frozen stocks. We cultured cardiomyocytes by each conditions, we considered condition of mitochondrial activity evaluation.
Other case studies
-
Pharmaceutical company
Inducing differentiation into cardiomyocytes from iPS cell-derived patient cells.
-
University
Development of obesity model medium using liver cell lines.
-
Ac.ademic research institute
Examination of functional improvement of myocardial 3D culture model using Myoridge original maturation medium.
-
Chemical manufacturer
Development of a method for evaluating drug responsiveness of cardiomyocytes using a new culture plate.
-
Chemical manufacturer
Development of media for drug evaluation.
-
IT company
Development of medium for primary culture of tumor cells derived from cancer patients.
List of Achievements in culture medium development
| Cell type | Results |
|---|---|
| iPS cell-derived cardiomyocytes | Achieving completely protein-free medium using small molecules and improving myocardial purity |
| iPS cells | Developed a low-cost medium with improved growth and adhesion properties |
| mesenchymal stem cells (Derived from bone marrow, fat and umbilical cord) | Serum-free, suppresses cell aging and achieves high proliferation and low cost. |
| T Cells (Cell line、derived from PBMC) |
Serum-free medium that can control CD4/CD8, improves Tregs and stem cell memory markers. |
| Car-T cells | Increased cell survival rate after introduction of Car gene, improved stem cell memory marker, serum free |
| antibody producing cells (B cells) | Serum-free for both human and mice, equivalent growth rate compared to commercially available media |
| vascular endothelial cells | Lower cost (less than 1/2) and equivalent growth rate (1.2 times or more) compared to commercially available media |
| Cancer cells | Lower cost (less than 1/2) and equivalent growth rate (1.2 times or more) compared to commercially available media, Serum-free |
| Blood related cells | Achieved serum-free and equivalent growth performance compared to commercially available media |
| Fibroblast | Achieved serum-free and equivalent growth performance compared to commercially available media. Developed for cell sheeting. |
| C2C12 cells (mouse myoblasts) |
One of the model cells for cultured meat cells, high proliferative, serum-free, low cost (less than 1/2), and improved muscle cell differentiation efficiency. |
